NOTE: This is from the biological report on the status of Atlantic Salmon - see Table of Contents and News Release for additional information.
SECTION 3: BIOLOGICAL INFORMATION
3.1 LIFE HISTORY
Atlantic salmon have a relatively complex life history that includes spawning and juvenile rearing in rivers to extensive feeding migration on the high seas. As a result, Atlantic salmon go through several distinct phases in their life history that are identified by specific behavioral and physiological changes and changes in habitat types (Figure 3.1). The following sections detail the life history typical of Atlantic salmon originating from U.S. rivers, during the periods spent in riverine and marine habitats. General descriptions of the life history of Atlantic salmon can be found in MacKenzie and Moring (1988), Bley and Moring (1988), Stanley and Trial (1995), and Baum (1997).3.1.1 Riverine Habitat
Adult Atlantic salmon ascend the rivers of New England beginning in the spring, continuing into the fall with the peak occurring in June. According to Baum, historically, the majority of the Atlantic salmon in the Penobscot, Dennys, East Machias, Narraguagus, Kennebec, Androscoggin and Saco Rivers entered freshwater between May and mid July and were therefore called "early run", whereas the majority of those returning to the St. Croix, Machias, and Ducktrap Rivers entered freshwater after mid- July and were called "late run". Some rivers such as the Sheepscot and Pleasant had both an early run and late run of Atlantic salmon (Baum 1997). Salmon that return early in the spring spend nearly five months in the river before spawning, seeking cool deep pools during the summer months. Homing to natal streams is thought to be facilitated with olfactory stimuli (Stasko et al. 1973).Straying rates (fraction of fish found in rivers other than their natal rivers) for Maine Atlantic salmon were estimated based on a tagging study of 1.5 million Penobscot River hatchery fish. Only 1-2% of these fish was found to enter a river other than the Penobscot (Baum 1997). Once an adult salmon enters a river, rising river temperatures and water flows stimulate upstream migration. However, water temperatures above 22.8o C or dissolved oxygen concentrations below 5 ppm can curtail movement (DeCola 1970).
When a salmon returns to its home river after two years at sea (called a two sea winter or 2SW fish) it is approximately 75 cm long and weighs approximately 4.5 kg. Some salmon, typically males, return after only one year at sea (1SW fish) at a smaller size and are termed "grilse". Occasionally, a large 3SW salmon is found among returning adults. In Maine, 95-98% of the grilse is male while 55-75% of the older fish returning is female (Baum 1997). The ranges are provided as a result of annual variation. For the period of 1970 to 1998, approximately 3% of the wild origin returning adults to the seven DPS rivers were 1 SW fish (USASAC 1999). Once in freshwater, adult salmon cease to feed during their up-river migration, and darken in color. Spawning occurs in late October through November.
Approximately 20% of Maine Atlantic salmon return to the sea immediately after spawning, the majority overwinter in the river and return to the sea the following spring (Baum 1997). A spawned salmon in freshwater is called a kelt or black salmon. Upon returning to salt water, the kelt resumes feeding and recovers its silver color. If the salmon, now a rejuvenated "bright" fish, should be among the minority to survive another 1-2 years at sea, it will return to its home river as a "repeat spawner". Thus, a spawning run of salmon may include several age groups, insuring some level of genetic exchange between generations.
Preferred spawning habitat is a gravel substrate with adequate water circulation to keep the buried eggs well oxygenated (Peterson 1978). Water depth at spawning sites is typically 30 cm to 61 cm and water velocity averages 60 cm per second (Beland 1984). Spawning sites are often located at the downstream end of riffles where water percolates through the gravel or where upwellings of groundwater occur (Danie et al. 1984). The optimal water temperature during the spawning period ranges from 7.2o C to 10.0o C (Jordan and Beland 1981; Peterson et al. 1977). The female moves her tail back and forth to create a depression in the gravel, called a redd, where she deposits eggs. One or more males fertilize the eggs as they are deposited in the redd (Jordan and Beland 1981). Redds in Maine average 2.4 m long and 1.4 m wide (Baum 1997). The female then continues digging upstream of the deposition site, burying the fertilized eggs. In Maine rivers, eggs are buried 12-20 cm on average and on average 240 eggs are deposited per habitat unit (one habitat unit equals 100 square meters of general habitat) (Baum 1997). A single female may create several redds before depositing all her eggs. Female anadromous Atlantic salmon produce a total of 1,500 to 1,800 eggs per kilogram of body weight. An average 2SW Maine Atlantic salmon produces 7,200 eggs. Weight loss in females ranges from 25-45% during spawning.
The eggs hatch in late March or April into a life stage that is called an alevin or sac fry. Alevins remain in the redd for about six weeks and are nourished by their yolk sac. When the alevins emerge from the gravel, about mid-May, and begin active feeding they are termed fry. Studies of fry in Maine reveal that the majority emerge from redds at night (>95%)(Baum 1997). Survival from eggs to the fry stage in Maine rivers has been reported to range from 8-35% (Jordan and Beland 1981; Meister, 1962; Baum 1997). Survival rates are affected by stream gradient, overwintering temperatures and water flows, and the level of predation and competition (Bley and Moring 1988). Within days, the fry enter the parr stage, indicated by vertical bars (parr marks) visible on their sides. These marks act as camouflage (Jones 1959). Survival from fry to parr ranges from 28-44% (Baum 1997). Parr measure from 4-10 cm in length and weigh between 10 and 100 grams (Baum 1997). During their early life in the river, salmon seek the cover provided by rocks and vegetation (Baum 1997).
Parr prefer areas with adequate cover, water depths ranging from approximately 10 cm to 60 cm, water velocities between 30 and 92 cm per second, and water temperature near 16o C (Beland 1984). A territorial instinct, first apparent during the fry stage, grows more pronounced during the parr stage, and the parr actively defend territories (Danie et al. 1984; Mills 1964; Kalleberg 1958; Allen 1940). Some male parr become sexually mature and can successfully participate in spawning with sea-run adult females. These males are referred to as "precocious parr". Water temperature (Elliot 1991), parr density (Randall 1982), photoperiod (Lundquist 1980), and the level of competition and predation (Hearn 1987, Fausch 1986), as well as food supply, influence the growth rate of parr. Maine Atlantic salmon rivers produce from five to ten parr per unit of habitat (Baum 1997). Juvenile Atlantic salmon feed on larvae of mayflies and stoneflies, chironomids, caddisflies and blackflies, aquatic annelids and mollusks as well as numerous terrestrial invertebrates that fall into the river (Scott and Crossman 1973).
In a parr's second or third spring, when it has grown to 12.5-15 cm in length, physiological changes occur that also result in visible morphological and behavioral changes (Schaffer and Elson 1975). This process, called "smoltification", prepares the parr for migration to the ocean and life in salt water. In Maine, the majority of parr remain in freshwater for two years (80%), while the balance remain for three years (Baum 1997). Survival from the parr to the smolt stage has previously been estimated to range from 35-55% (Baum 1997). Research in the Narraguagus River, however, demonstrated a 99% probability that survival was less than 30% (Kocik 1998). The juvenile fish loses its parr markings and its body becomes streamlined and silvery with a pronounced fork in the tail. Orientation in the water column changes from facing upstream to downstream. The biochemical and physiological changes that occur during smoltification prepare the fish for the dramatic change in osmoregulatory needs that come with the transition from a fresh to a salt water habitat (Bley 1987; Farmer et al. 1977; Hoar 1939; USFWS 1989; Ruggles 1980). As smolts migrate from the rivers from April to June, they tend to travel near the water surface and contend with changes in water temperature, pH, dissolved oxygen, pollution levels, and predation. Maine smolts range in size from 13-23 cm (Baum 1997). Most smolts in New England rivers enter the sea during May and June to begin their ocean migration. Baum provides the "rule-of-thumb" that Maine salmon rivers produce 19 fry/unit, resulting in 6 parr/unit and ultimately 3 smolts/unit (1997). Survival from fry to smolt, based on results from hatchery fry plantings, is reported by Bley and Moring (1988) to range from about 1% to 12% and survival from egg to smolt stage is reported by Baum (1997) to be approximately 1.25%. Martin (1995) references Elliot (1991) in reporting lethal water temperatures for salmon in freshwater as 27.8° C for seven days and 32.9° C for 10 minutes.
3.1.2 Marine Habitat
The marine life history of Atlantic salmon of U.S. origin is not as well understood as the freshwater phase. A major obstacle to the study of Atlantic salmon in the marine environment has been the relatively low density of salmon over the extended geographic range in the ocean (Figure 3.1.2) (Hislop and Shelton 1993). However, in the last ten years there has been substantial progress in understanding the marine ecology and population dynamics of Atlantic salmon. Central to this progress has been the work of assessment committees such as the U.S. Atlantic Salmon Assessment Committee (USASAC), the International Council for the Exploration of the Sea (ICES) Working and Study Groups (the North American Salmon Study Group (ICES-NASSG) and the North Atlantic Salmon Working Group (ICES-NASWG). Within the framework of providing scientific advice to the multinational North American Salmon Conservation Organization (NASCO), basic understanding of the marine ecology of the species has been advanced (Windsor and Hutchinson 1994).
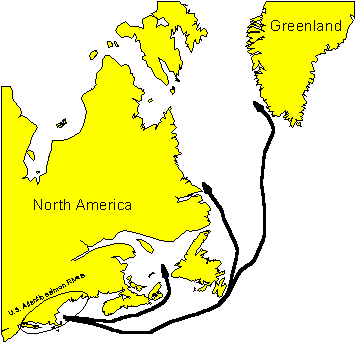
Figure 3.1.2: Generalized marine migration routes of U.S. origin Atlantic salmon
Much of our knowledge of U.S. Atlantic salmon at sea has been derived from marking and tagging studies of fish stocked in the Connecticut, Merrimack, and Penobscot Rivers. Over the history of the U.S. program, marking has progressed from fin clipping (1942-1962), to Carlin tags (1962-1992), to coded-wire tags (CWT) from 1985 to the present (Meister 1984; NASCO 1993b). From these investigations, scientists have gained a better understanding of the movement and exploitation of U.S. Atlantic salmon at sea (Meister 1984; NASCO 1993b; Reddin and Friedland 1993). Scientists have also discovered correlations between natural mortality in the marine environment and abiotic factors, particularly sea surface temperature (SST) (Scarnecchia 1984a, 1984b; Martin and Mitchell 1985; Scarnecchia et al. 1989; Friedland and Reddin 1993; Friedland et al. 1993). Additional studies that have directly sampled Atlantic salmon in the ocean have also provided important insights (Dutil and Coutu 1988; Reddin 1988; Reddin et al. 1991; Ritter 1989). While our understanding of the marine ecology of Atlantic salmon is still incomplete, these investigations have helped discern movements, exploitation, and population dynamics (Meister 1984; NASCO 1993b; Reddin and Friedland 1993; Friedland et al. 1993).
Atlantic salmon of U.S. origin are highly migratory (Figure 3.1.2), undertaking long marine migrations from the mouths of U.S. rivers into the Northwest Atlantic Ocean, where they are distributed seasonally over much of the region (Reddin 1985). The marine phase starts with smoltification and subsequent migration through the estuary of the natal river. Smolt movement in the predominantly freshwater sections of the estuary is relatively passive, progressing seaward on ebb tides and neutral or upstream on flood tides (Fried et al. 1978; Thorpe et al. 1981). As smolts enter the more saline portions of the estuary, their movements are more directed and less affected by tides. They move rapidly seaward at speeds averaging two body lengths per second (La Bar et al. 1978).
Upon completing the physiological transition to salt water, the post-smolts grow rapidly and have been documented to move in small schools and loose aggregations close to the surface (Dutil and Coutu 1988). The post-smolt stage is probably the least understood period during the life history of Atlantic salmon; recaptures of post-smolts are limited because Atlantic salmon fisheries target older, larger fish. Most of the U.S.- origin post-smolt tag recoveries have come from incidental catch in herring and mackerel weirs in the Bay of Fundy and South Shore of Nova Scotia during July (Meister 1984). Tag recoveries from sea-bird colonies have indicated that U.S. post-smolts are also present off eastern Newfoundland by August (Montevecchi et al. 1988; Reddin and Short 1991). Upon entry into the nearshore waters of Canada, the U.S. post-smolts become part of a mixture of stocks of Atlantic salmon from various North American streams. Post-smolts in the northern Gulf of St. Lawrence stay nearshore for much of the first summer. Decreasing nearshore temperatures in autumn appear to trigger offshore movements of these fish (Dutil and Coutu 1988). Post-smolts also occur off the Grand Bank and further North in the Labrador Sea during the summer and autumn (Reddin 1985; Reddin and Short 1991; Reddin and Friedland 1993), where the North American stock complex intermixes with fish from Europe and Iceland. The U.S. stocks of Atlantic salmon thus become a small portion of a larger mixed-stock complex. The U.S. contribution to the stock complex has probably always been relatively low because the basin wide production is modest.
Upon entry to the marine environment, post-smolts appear to feed opportunistically, primarily in the neuston (near the surface). Their diet includes invertebrates, amphipods, euphausiids, and fish (Hislop and Youngson 1984; Jutila and Toivonen 1985; Fraser 1987; Hislop and Shelton 1993). As post-smolts grow, fish become an increasingly dominant component of their diet. Atlantic salmon post-smolts, because of their small size, are preyed upon by cod, whiting, cormorants, ducks, terns, gulls, and many other opportunistic predators (Hvidsten and Mokkelgjerd 1987; Gunnerod et al. 1988; Hvidsten and Lund 1988; Montevecchi et al. 1988; Hislop and Shelton 1993). Predation rates are difficult to estimate because of the wide spatial and temporal distribution of Atlantic salmon at low densities and the large number and variety of potential predators.
Information on the overwintering of post-smolts at sea is limited. Based upon analyses of scales, it appears that growth is minimal during this time (Friedland et al. 1993). The location of stocks during the winter is uncertain, but high spring catch rates of one-sea-winter (1SW) Atlantic salmon in the Labrador Sea caused Reddin and Friedland (1993) to hypothesize that post-smolts overwinter in the southern Labrador Sea. It is also likely that some component of the North American stock complex overwinters in the Bay of Fundy (Reddin and Friedland 1993). Direct sampling during the winter months would be helpful in gaining a better understanding of post-smolt Atlantic salmon distribution in the North Atlantic.
Most U.S. origin salmon spend two winters (2SW) in the ocean before returning to streams for spawning. Fish that return to freshwater after only one year at sea are called grilse, whereas those that spend multiple years at sea are called salmon. The 1SW and multi-sea-winter (MSW) Atlantic salmon are thought to behave similarly to the post-smolts, moving through the top six meters of the water column (Reddin 1985). Aggregations of Atlantic salmon may still occur after the first winter, but most evidence indicates that they travel individually (Reddin 1985). At this stage, Atlantic salmon primarily eat fish (piscivorous), feeding upon capelin (Mallotus villosus), herring (Alosa sp.), and sand lance (Ammodytes sp.) (Hansen and Pethon 1985; Reddin 1985; Hislop and Shelton 1993). Their increasing size makes them decreasingly vulnerable to predation by smaller piscivores that feed upon post-smolts. Although most Atlantic salmon are caught near the surface, several benthic predators such as gadids, skates, and Greenland shark (Simniosus microcephalus) are known to eat them at sea (Hislop and Shelton 1993). This suggests that Atlantic salmon do spend some time in deeper waters and indicates the need for further behavioral studies.

